MATE 664 Lecture 11
Diffusion in Defect and Material Imperfections
- Slides 👉 Open presentation🗒️
- PDF version of course note 👉 Open in pdf
- Handwritten notes 👉 Open in pdf
Recap of Lecture 10
Key ideas from last lecture:
- Diffusion equations for solids
- Link Einstein’s equation to Arrhenius equation
- Emergence of activation enthalpy / entropy
- Vacancy formation free energy and diffusion
Learning Outcomes
After this lecture, you will be able to:
- Apply the free energy-dependent diffusion equations to systems with defects and material imperfections
- Understand how intrinsic and extrinsic defect in ionic materials are formed
- Analyze intrinsic / extrinsic diffusion regimes in ionic materials
- Understand material imperfections as the diffusion shortcuts
What Does Diffusion In Real Materials Look Like?
- Real solids are not perfect crystals
- Defects control transport properties
- Ionic diffusion shows rich temperature dependence
- Same Einstein framework, new physics inside
Base Line: Vacancy-Mediated Diffusion In Metals
- Diffusion = random walk of atoms via vacancies
- Diffusivity related to both vacancy density (controlled by \(G_v^f\)) and vacancy jumping (controlled by \(G_v^m\))
Defect-Mediated Diffusion In Ionic Crystals
- Defects in ionic crystals are more complex than in metal
- Charge neutrality has to be conserved
- More than one single defect species are involed
- In other words, defects always come in pairs in ionic solids
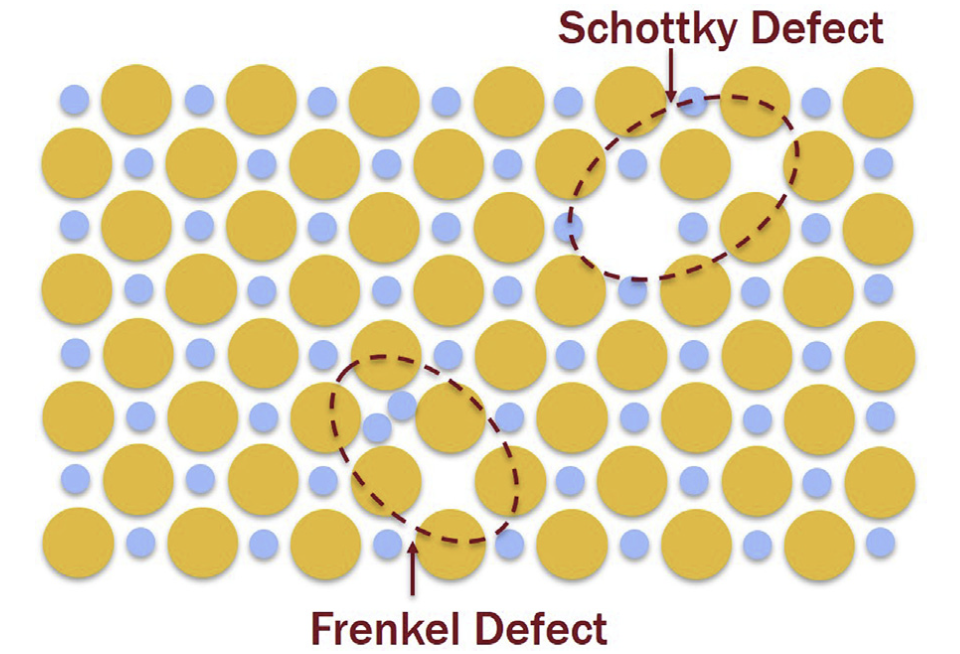
Schottkey and Frenkel Defects In Ionic Crystals
- Shottkey defects: missing both positive and negative species
- Frenkel defects: one charged species moved to other site
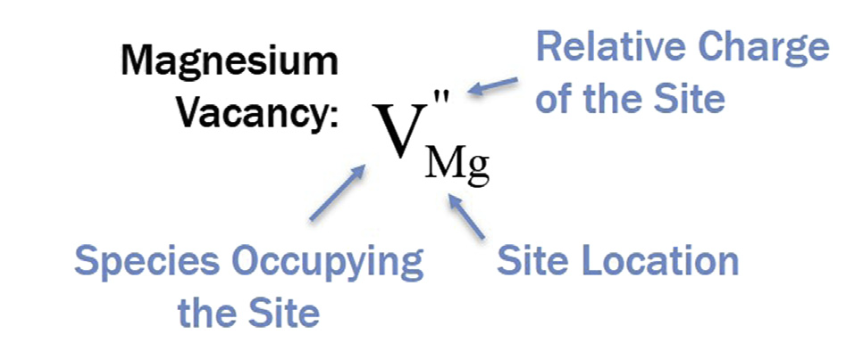
Ionic Defects: Kröger-Vink (KV) notation
- Formula \(X^Z_Y\)
- X = what is at the site (Element or Vacancy)
- Y = what site is defective (Element or i)
- Z = effective charge at the site (• = +; ’= –)
KV Notation For Schottky Defects
Example: Schottky defects in MgO (anion + cation vacancies)
Formation reaction
\[ \text{null} \rightarrow V_{\mathrm{Mg}}^{''} + V_{\mathrm{O}}^{\bullet\bullet} \]
Equilibrium condition
- \(G_S^f\): formation free energy for Schottky defects (per reaction)
\[ K_S = [V_{\mathrm{Mg}}^{''}][V_{\mathrm{O}}^{\bullet\bullet}] = \exp\!\left(-\dfrac{G_S^{f}}{kT}\right) \]
Charge neutrality
\[ [V_{\mathrm{Mg}}^{''}] = [V_{\mathrm{O}}^{\bullet\bullet}] \]
KV Notation For Frenkel Defects
Example: Frenkel pairs in LiF (Lithium escaping to interstitial sites)
Formation reaction
\[ \mathrm{Li}_{\mathrm{Li}}^{\times} \rightarrow V_{\mathrm{Li}}^{'} + \mathrm{Li}_i^{\bullet} \]
Equilibrium condition
- \(G_F^f\): formation free energy for Schottky defects (per reaction)
\[ K_F = [V_{\mathrm{Li}}^{'}][\mathrm{Li}_i^{\bullet}] = \exp\!\left(-\dfrac{G_F^{f}}{kT}\right) \]
Charge neutrality
\([V_{\mathrm{Li}}^{'}] = [\mathrm{Li}_i^{\bullet}]\)
Intrinsic vs Extrinsic Defects
- Intrinsic defects are those controlled by thermodynamics \(G_S^f\), \(G_F^f\) or \(G_V^f\)
- Extrinsic defects are those defects added to the ionic crystal via doping
Example of Extrinsic defects: CdCl\(_2\) in NaCl
Dopant incorporation reaction
\[ \mathrm{CdCl}_2 \rightarrow \mathrm{Cd}_{\mathrm{Na}}^{\bullet} + 2\,\mathrm{Cl}_{\mathrm{Cl}}^{\times} + V_{\mathrm{Na}}^{'} \]Extrinsic defect concentration
\[ [V_{\mathrm{Na}}^{'}]_{\text{ext}} = [\mathrm{Cd}_{\mathrm{Na}}^{\bullet}] = [\mathrm{CdCl}_2] \]Total vacancy concentration
\[ [V_{\mathrm{Na}}^{'}] = [V_{\mathrm{Na}}^{'}]_s + [V_{\mathrm{Na}}^{'}]_{\text{ext}} = \exp\!\left(-\dfrac{G_S^{f}}{2kT}\right) + [\mathrm{CdCl}_2] \]
Diffusivity of Ionic Species
- We can still use the vacancy exchange mechanism for the diffusivity of ionic species
- E.g. Na\(^+\) exchanges with its own vacancy \(V_{\mathrm{Na}}^{'}\)
- \([V_{\mathrm{Na}}^{'}]\) depends on the \(T\)-regime!
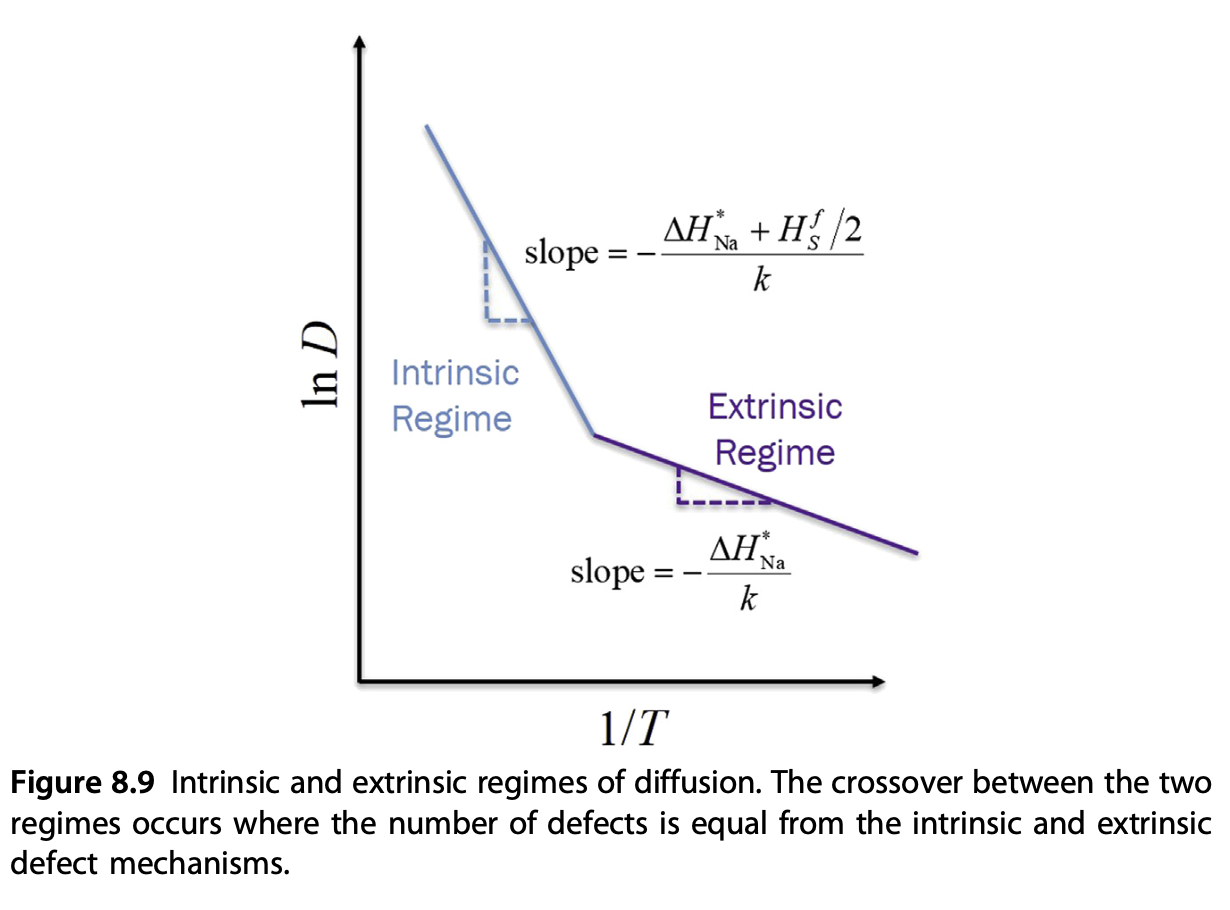
Intrinsic vs Extrinsic Regimes
- Extrinsic: vacancy dominated by doped materials
- Low-\(T\) regime (high \(1/T\))
- Intrinsic: vacancy dominated by thermal dissociation
- High-\(T\) regime (low \(1/T\))
Two-Regimes In The Arrhenius Plot
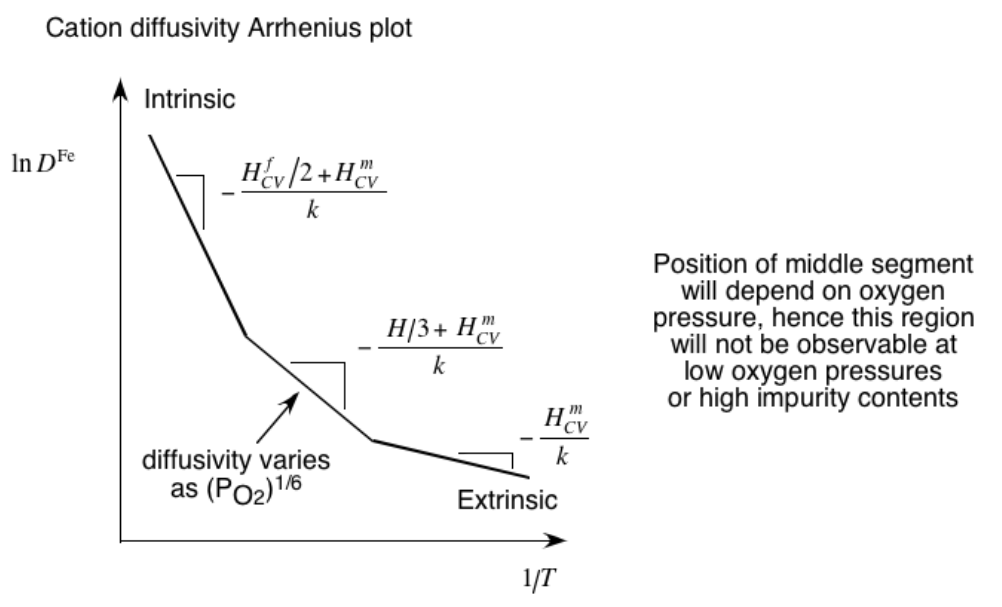
More Regimes In Arrhenius Plot
- Example: cation diffusion in FeO during oxidation
- Equilibrium depends on both \(G^f\) and \(p(O_2)\)
- Multiple regimes!
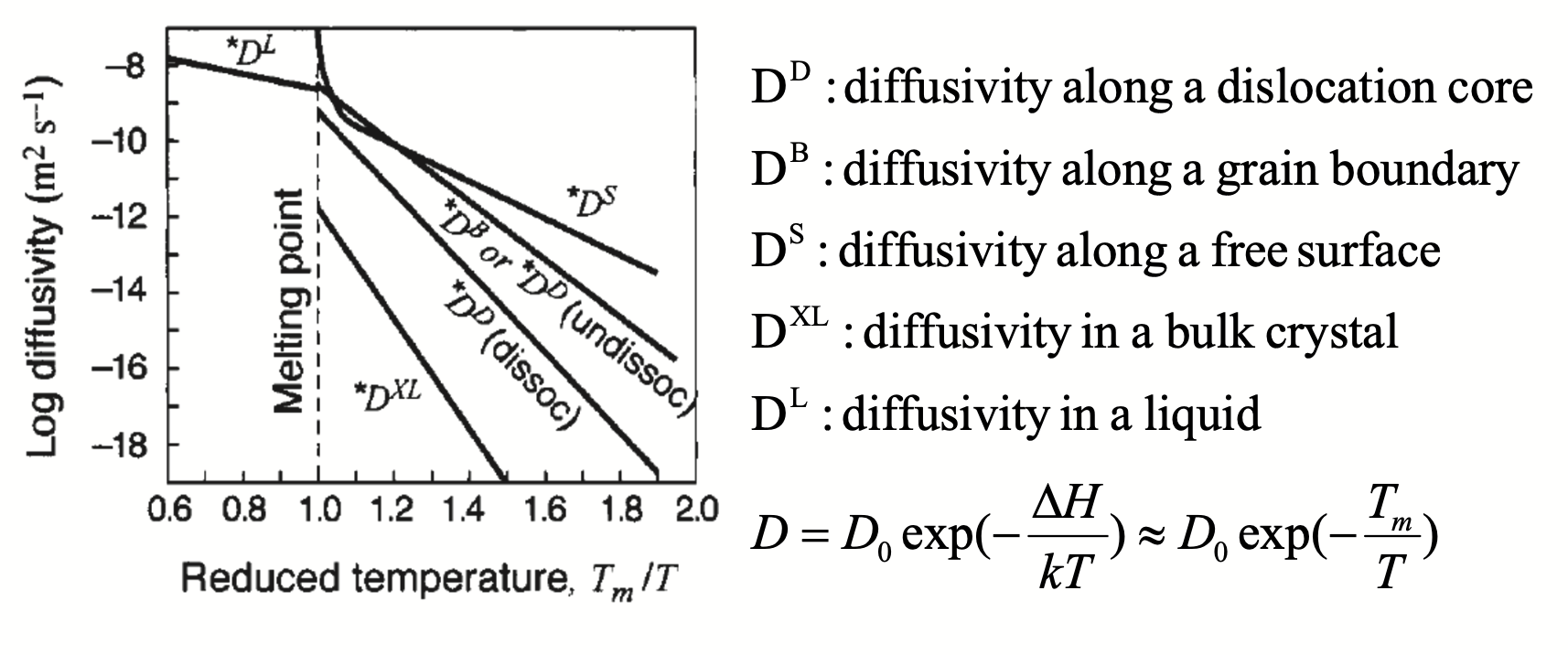
Multiple Regimes Diffusion In Polycrystalline Materials
Diffusion Paths At Crystal Imperfections
- Diffusion can occur along non-bulk pathways
- Typical crystal imperfections
- Grain boundary and interface diffusion: 2D
- Free surface diffusion: 2D
- Dislocation (pipe) diffusion: 1D
- Vacancy / defect: 0D
- Imperfections are associated with lower migration / activation energy!
- Think as “shortcuts” during diffusion
Imperfection 1: Grain Boundaries
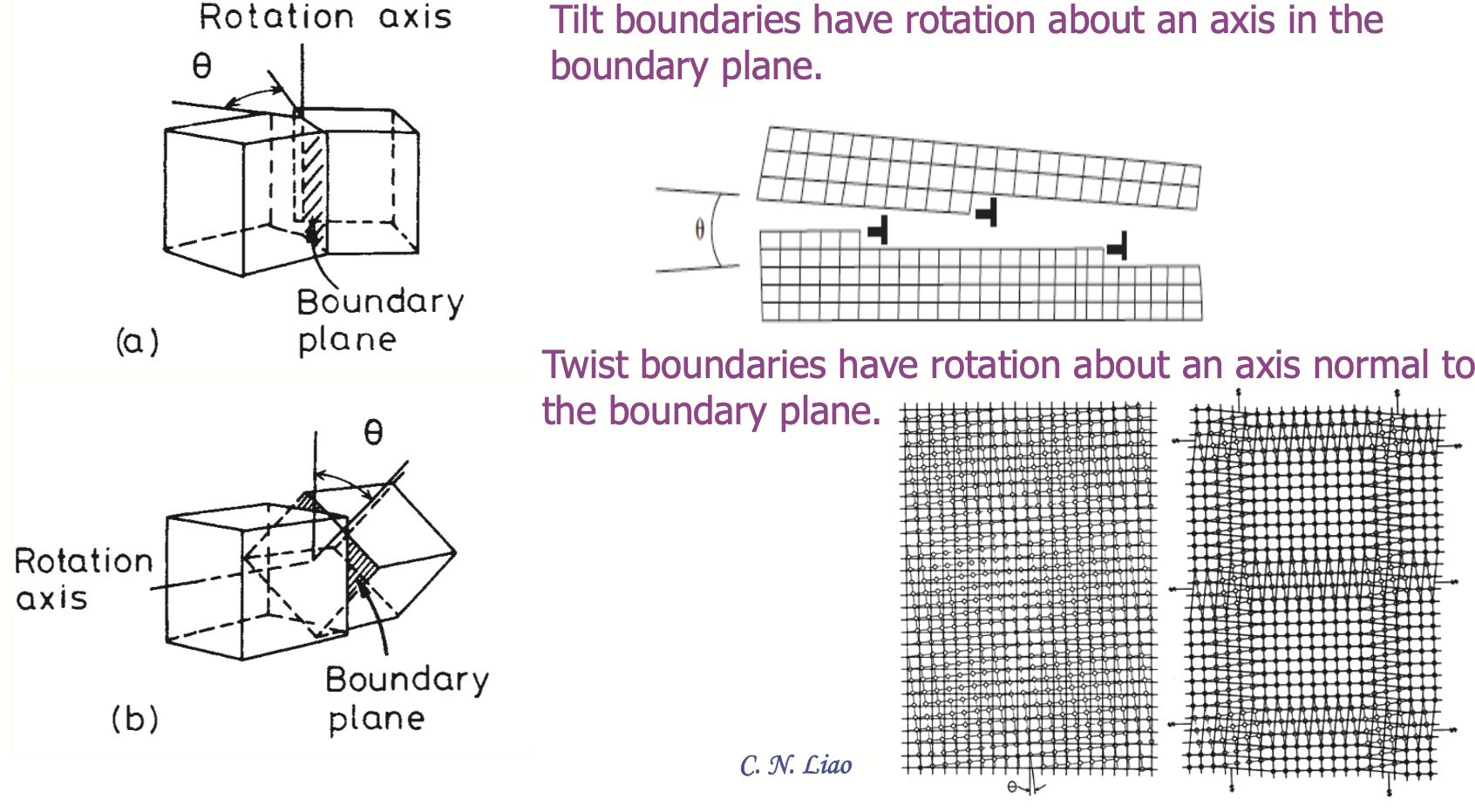
Grain Boundary Diffusion
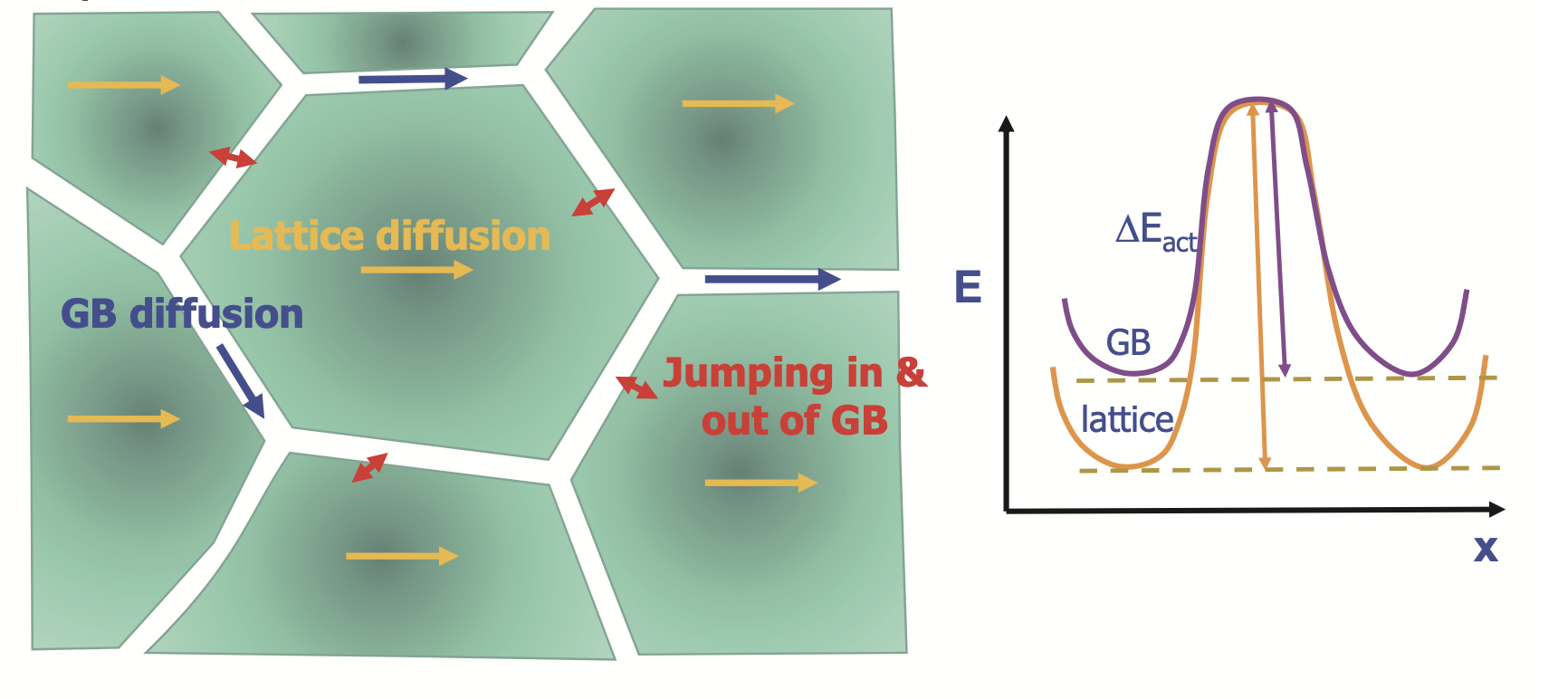
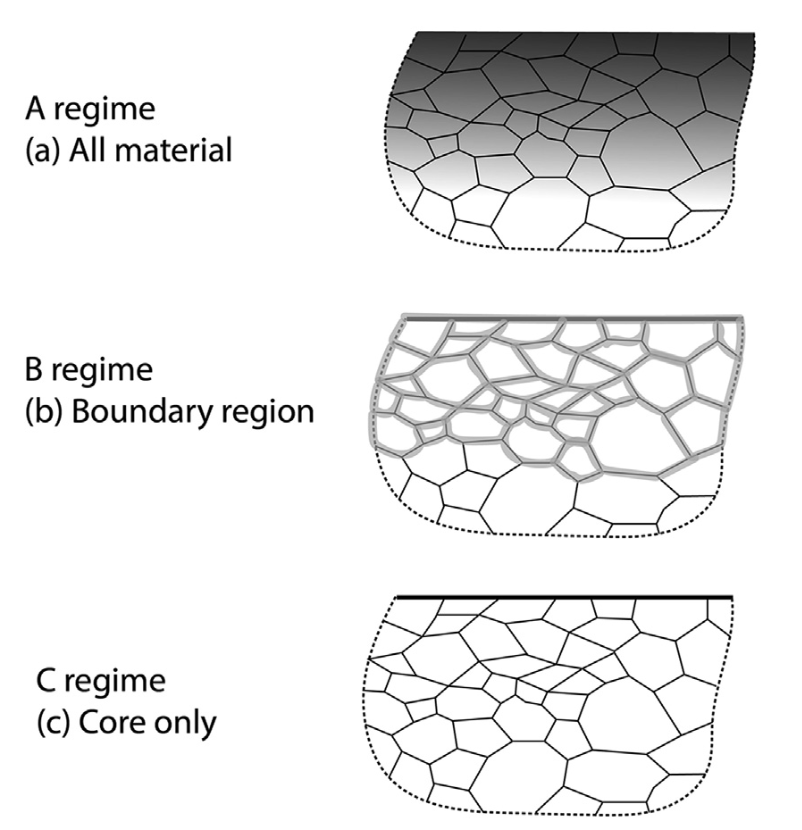
Harrison’s ABC Model For GB Diffusion
- A regime
- \(D_{XL} t > l^2\)
- \(D_B t > l^2\)
- fast diffusion everywhere
- \(D_{XL} t > l^2\)
- B regime
- \(D_{XL} t \gg l^2\)
- \(D_B t < l^2\)
- coupled short-circuit and bulk diffusion
- \(D_{XL} t \gg l^2\)
- C regime
- \(D_{XL} t < l^2\)
- \(D_B t < l^2\)
- diffusion confined to imperfections
- \(D_{XL} t < l^2\)
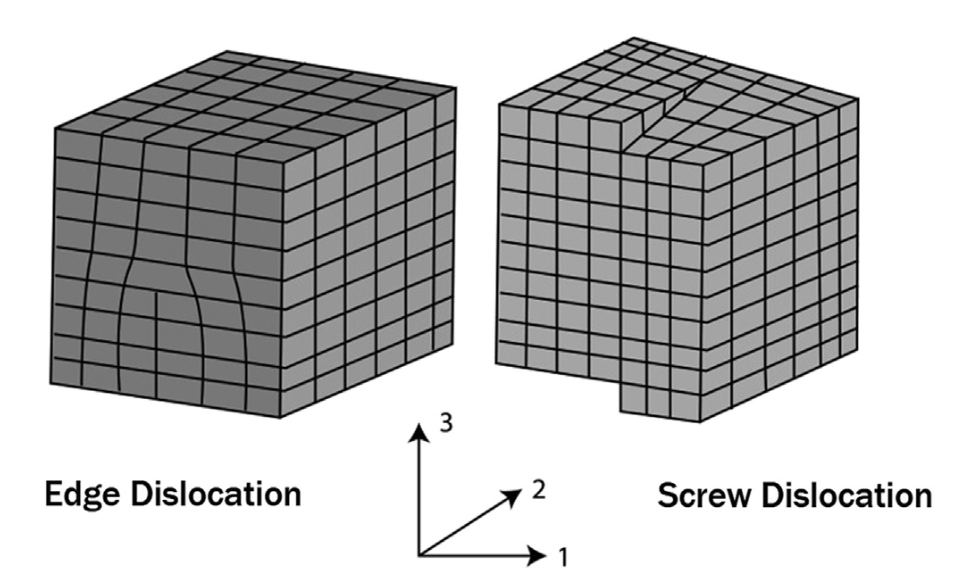
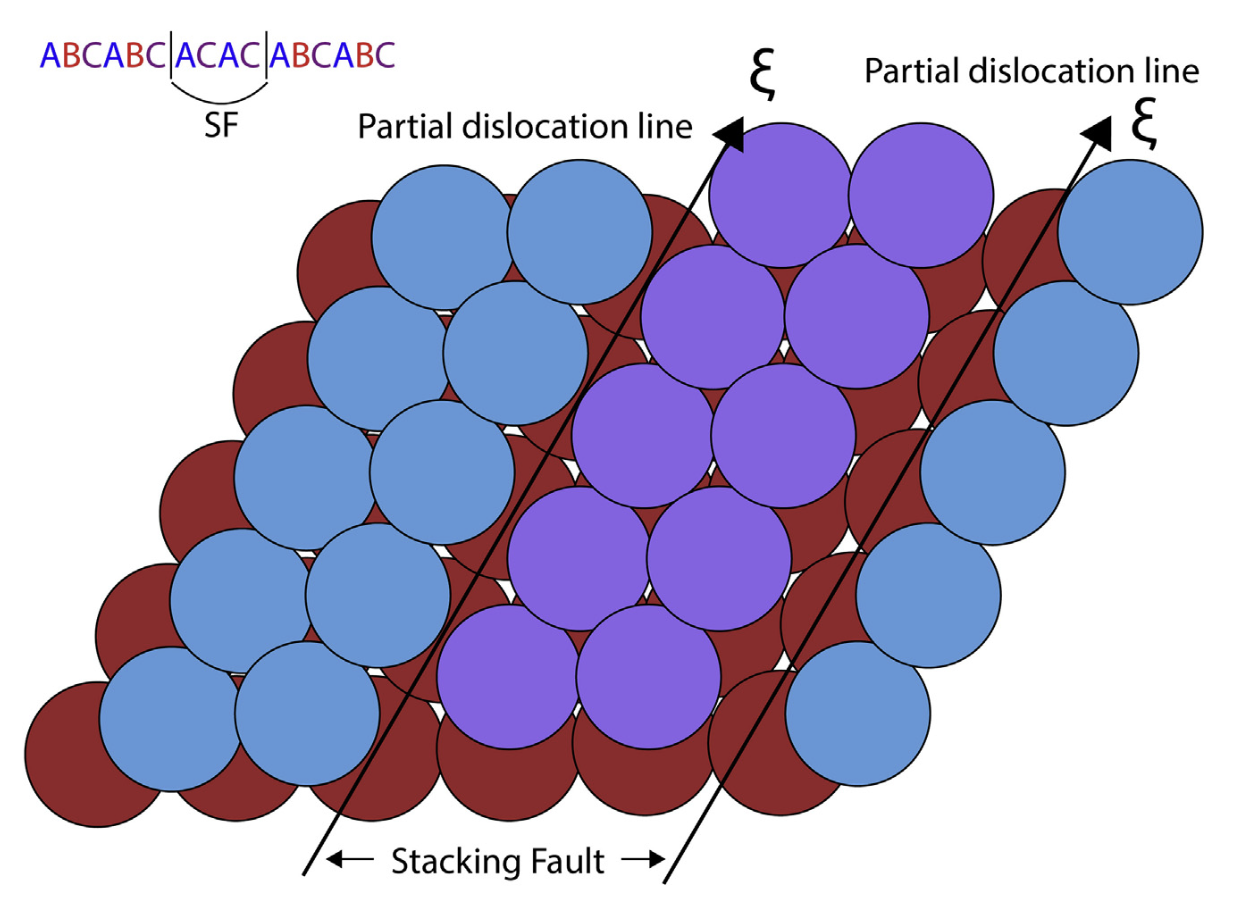
Dislocation Imperfections (Line Defect)
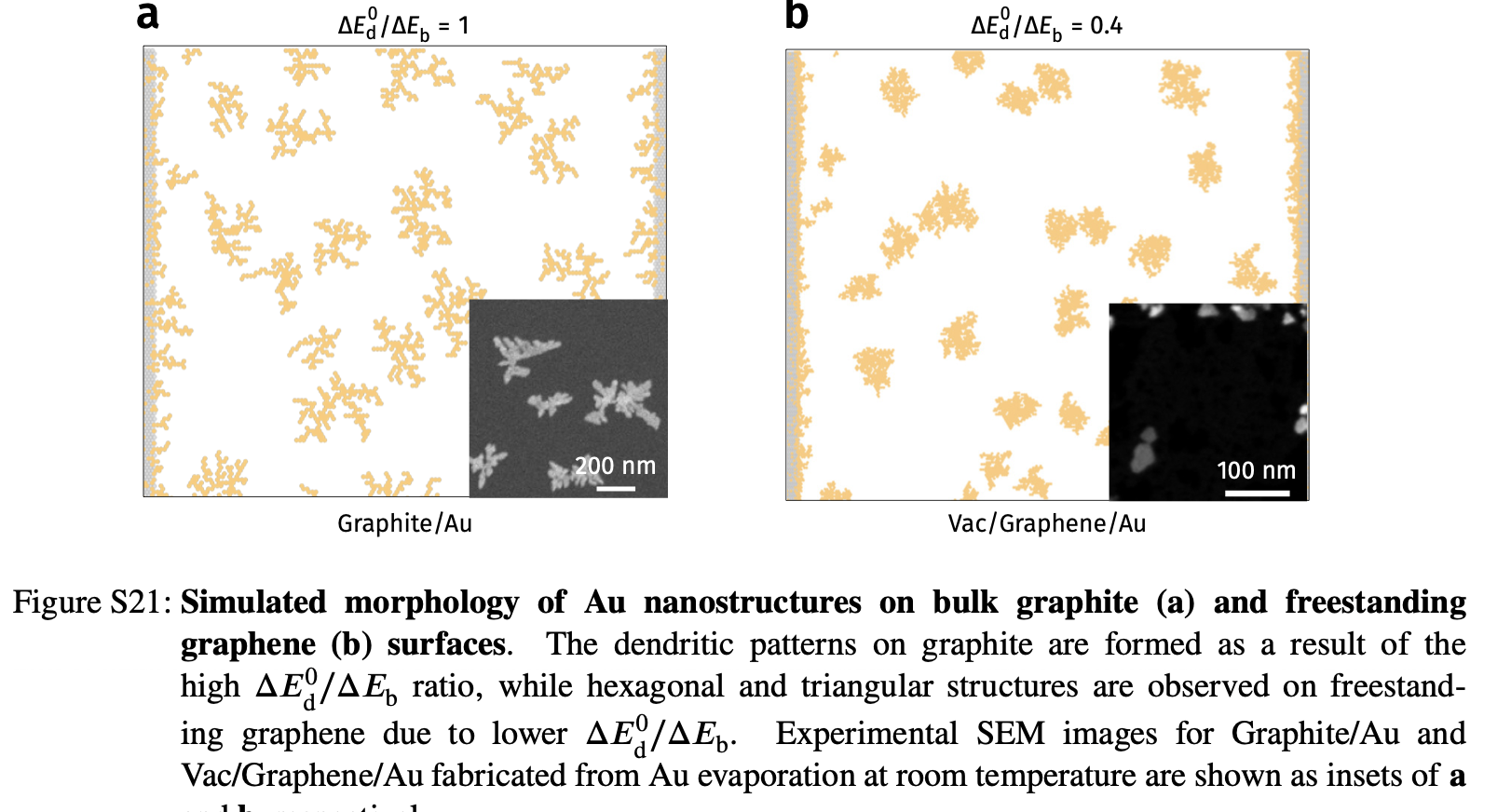
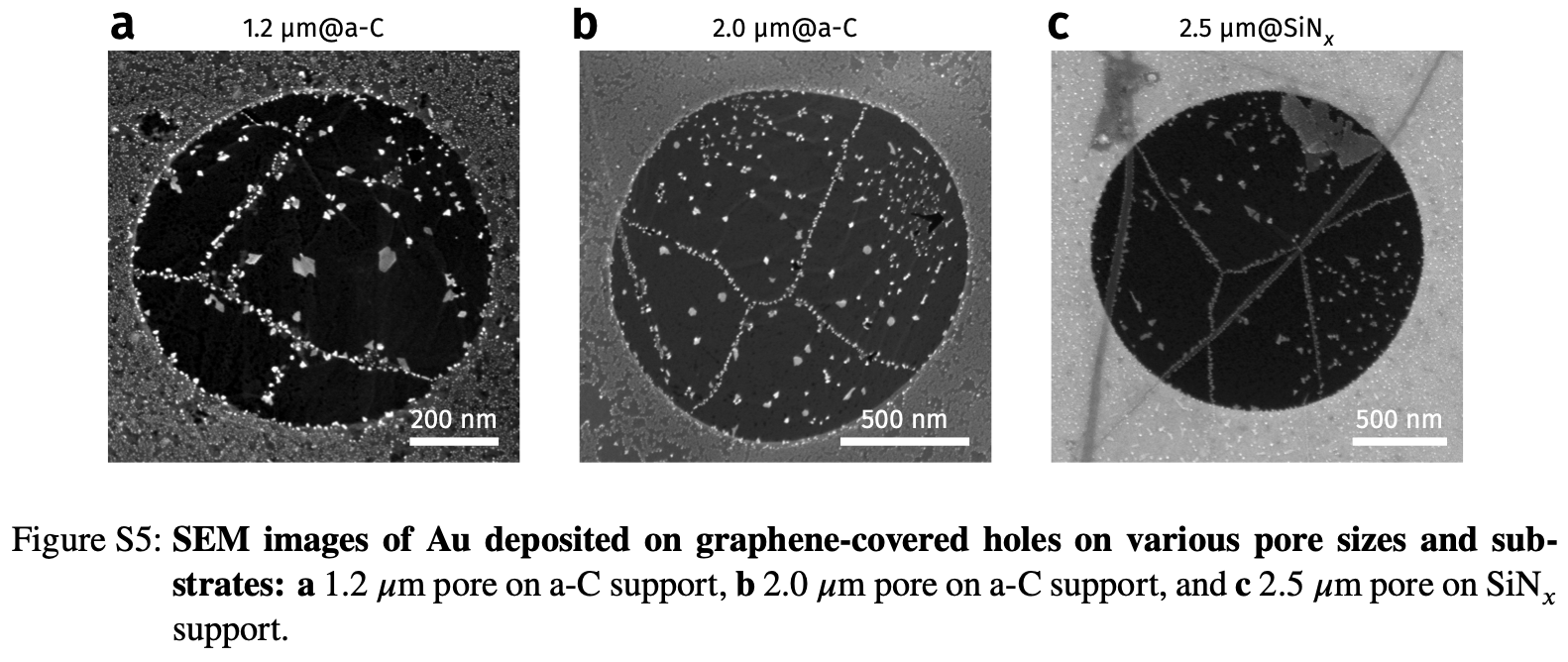
Example of Diffusion Along Imperfection: Deposition on Graphene
- See Vagli and Tian et al. Nat Commun 2025, 16, 7726.
- Diffusivity change on free graphene surface can be probed by deposition geometry!
Deposition on Graphene - Theoretical Simulations
- Kinetic Monte Carlo (kMC) assuming different diffusion barrier on imperfections
- Faster diffusion direction –> lower density
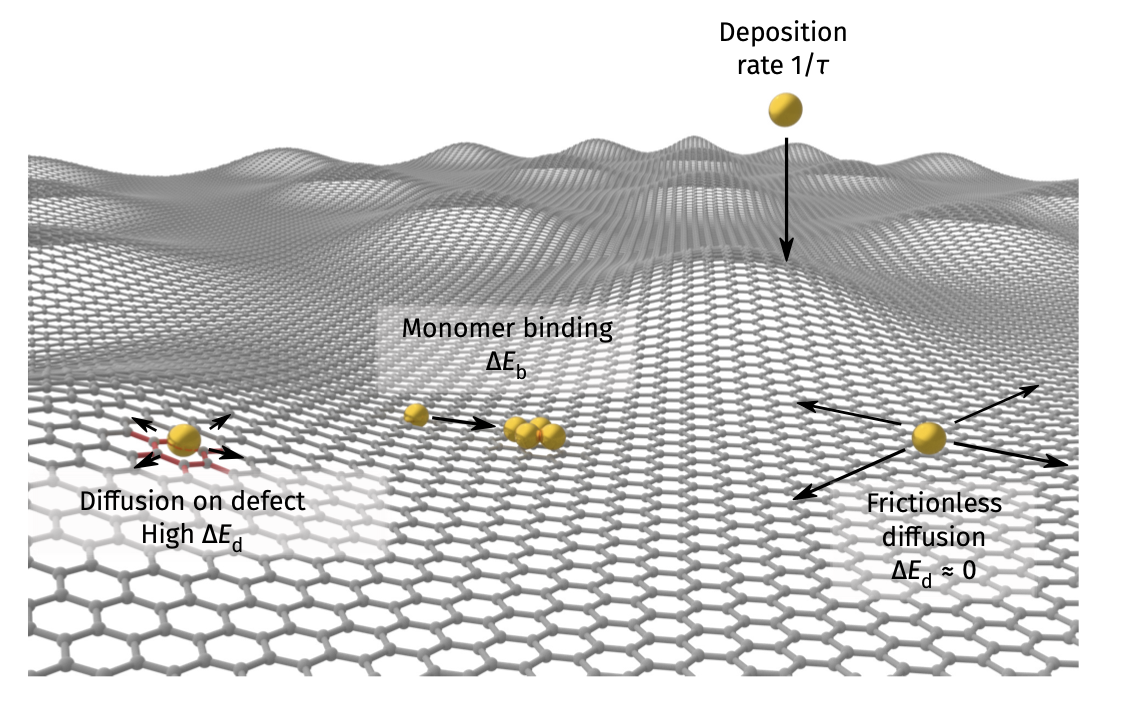
Deposition on Graphene - Theoretical Simulations
- Kinetic Monte Carlo (kMC) assuming different diffusion barrier on imperfections
- Faster diffusion direction –> lower density
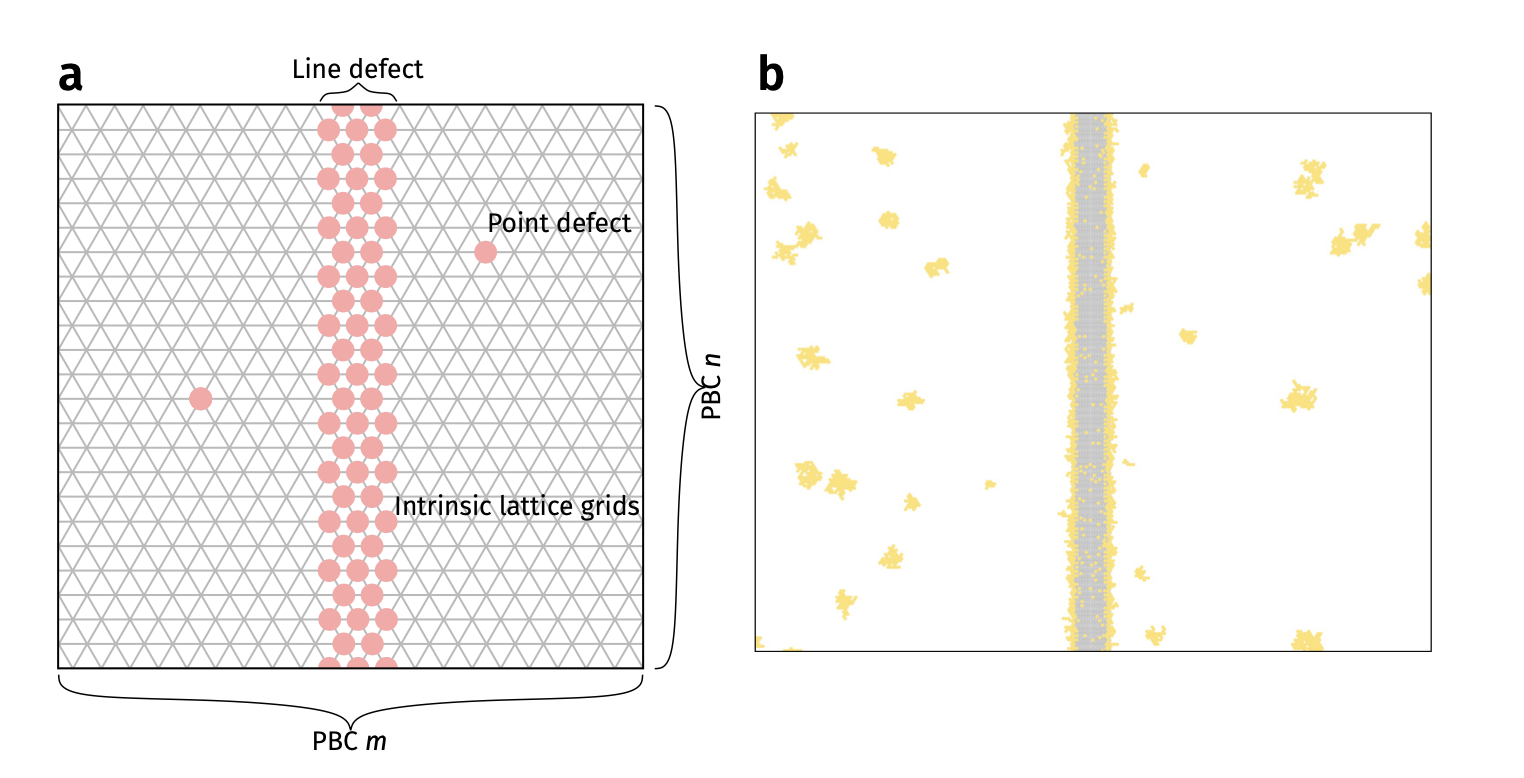
Deposition on Graphene - KMC vs Experiments