MATE 664 Lecture 01
Thermodynamics and Kinetics
- Slides 👉 Open presentation🗒️
- PDF version of course note 👉 Open in pdf
- Handwritten notes 👉 Open in pdf
Land Acknowledgement
The University of Alberta acknowledges that we are located on Treaty 6 territory, and respects the histories, languages, and cultures of the First Nations, Métis, Inuit, and all First Peoples of Canada, whose presence continues to enrich our vibrant community.
Learning Outcomes
After today’s lecture, you will be able to:
- Identify the key components of the course syllabus, content and grading schemes.
- Recall common interaction methods and resources available in the course.
- Recall basic concepts in thermodynamics and kinetics
- State assumptions of equilibrium
- Describe the influence of entropy in kinetic systems
Course Information
- Course: MAT E 664 – Kinetics of Materials
- Term: Winter 2026
- Lectures: Mon & Wed
- Time: 14:00 – 15:20
- Location: HC 2-14
Meet the Instructor
Office: DICE 12-245
Email: tian.tian@ualberta.ca
Office hour: by appointment
I joined CME in 2025 as assistant professor.
Research fields: machine learning, multiscale materials simulations, computational tools
Let’s enjoy learning together!
TAs & Seminar Sessions
- Teaching Assistant
- Hanlin Wang — hanlin7@ualberta.ca
- Course & Assignment Questions
- No formal seminar or lab sessions
- Questions related to course content or assignments
- Please book an appointment with the TA (and instructor) as needed
- Support Format
- One-on-one or small-group discussions
- Concept clarification and guidance
Course Grading
- Assignments: 25%
- 4 assignments (best 3 counted)
- Submission via Canvas
- Final Project: 30%
- Research-related topic
- In-class oral presentation
- Final Exam: 45%
- In person, open book
- Apr 20, 2026 · 1:00 p.m.
Details please see the course syllabus
Textbook and References
Our primary textbooks for this course are:
- Kinetics of Materials by R.W. Balluffi, S.M. Allen, and W.C. Carter.

- Materials Kinetics: Transport and Rate Phenomena by John C. Mauro.

What Will We Learn in MAT E 664 (1)?
Theory: irreversible thermodynamics & driving forces
\[ \text{Flux} = \text{Kinetic coefficients} \times \text{Driving Force} \]

What Will We Learn in MAT E 664 (2)?
Mass transport on solid material interfaces: \(v_{step} = \beta (c - c_{eq})\)

## What Will We Learn in MAT E 664 (3)?
Nucleation theory: \(r_{c} = -\dfrac{2\gamma}{\Delta G_v}\)

What Will We Learn in MAT E 664 (4)?
Spinodal Decomposition (Pattern Formation) \[ \dfrac{\partial c}{\partial t} = \nabla \cdot \left( M \nabla \left[ \dfrac{\partial f(c)}{\partial c} - \kappa \nabla^{2} c \right] \right) \]
Interaction Time!
We will use Wooclap in this course for real-time interactions.
Participation link 👉 https://app.wooclap.com/664L01?from=instruction-slide
Results to be published after the class
Thermodynamics vs. Kinetics
| Feature | Thermodynamics | Kinetics |
|---|---|---|
| Greek Name | Therme (heat) + dynamis (power) | Kinetikos (of motion) |
| Focus | Eventually: Predicts the final state | Rate: How fast a process occurs |
| General Form | Free energy change (\(\Delta G\)) | Reaction rates, flux, activation energy |
| Condition | Equilibrium | Non-equilibrium |
The True Meaning of Equilibrium
Equilibrium is a balance of time scales: \[ \tau_{\text{observation}} \gg \tau_{\text{process}} \]
- Thermodynamic descriptions are relevant when the observation time scale is much larger than the time scale of the processes reaching equilibrium.
- It’s about specific processes reaching a steady state, not necessarily the entire system.
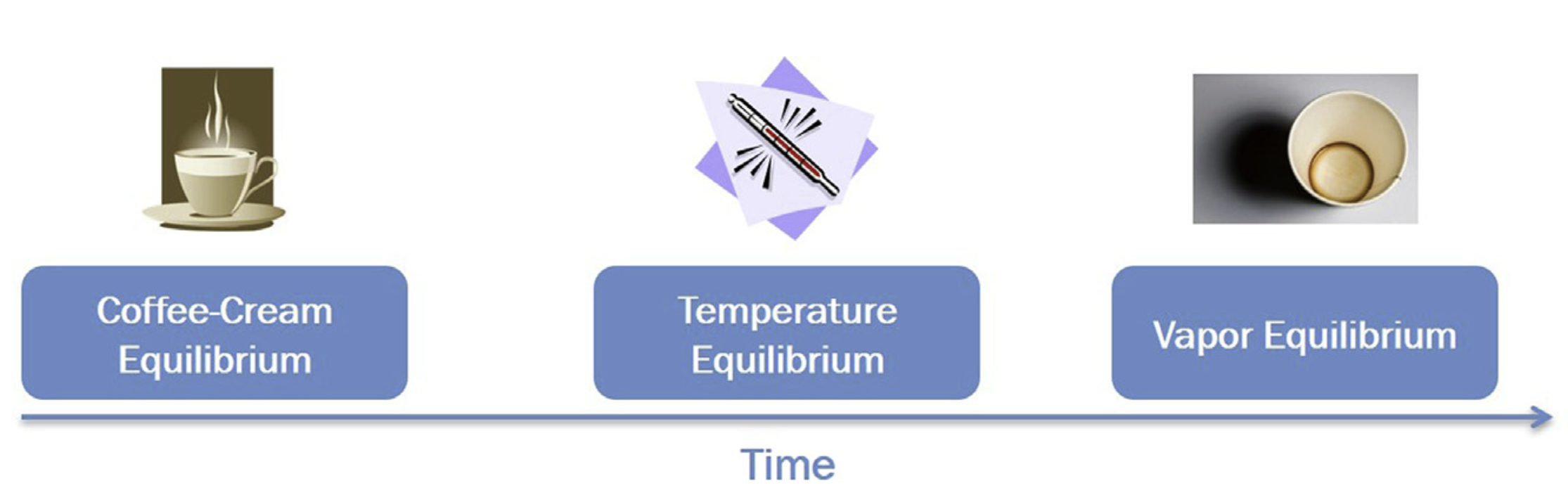
Kinetic Processes
Kinetic processes are distinct from thermodynamic equilibrium:
- Conditions: Occur away from equilibrium.
- Cause Require a thermodynamic driving force.
- Rate: Coupled with a rate parameter or coefficient.
Irreversible Thermodynamics is key to understanding these processes (Lecture 2).
Classical Thermodynamics Revisited
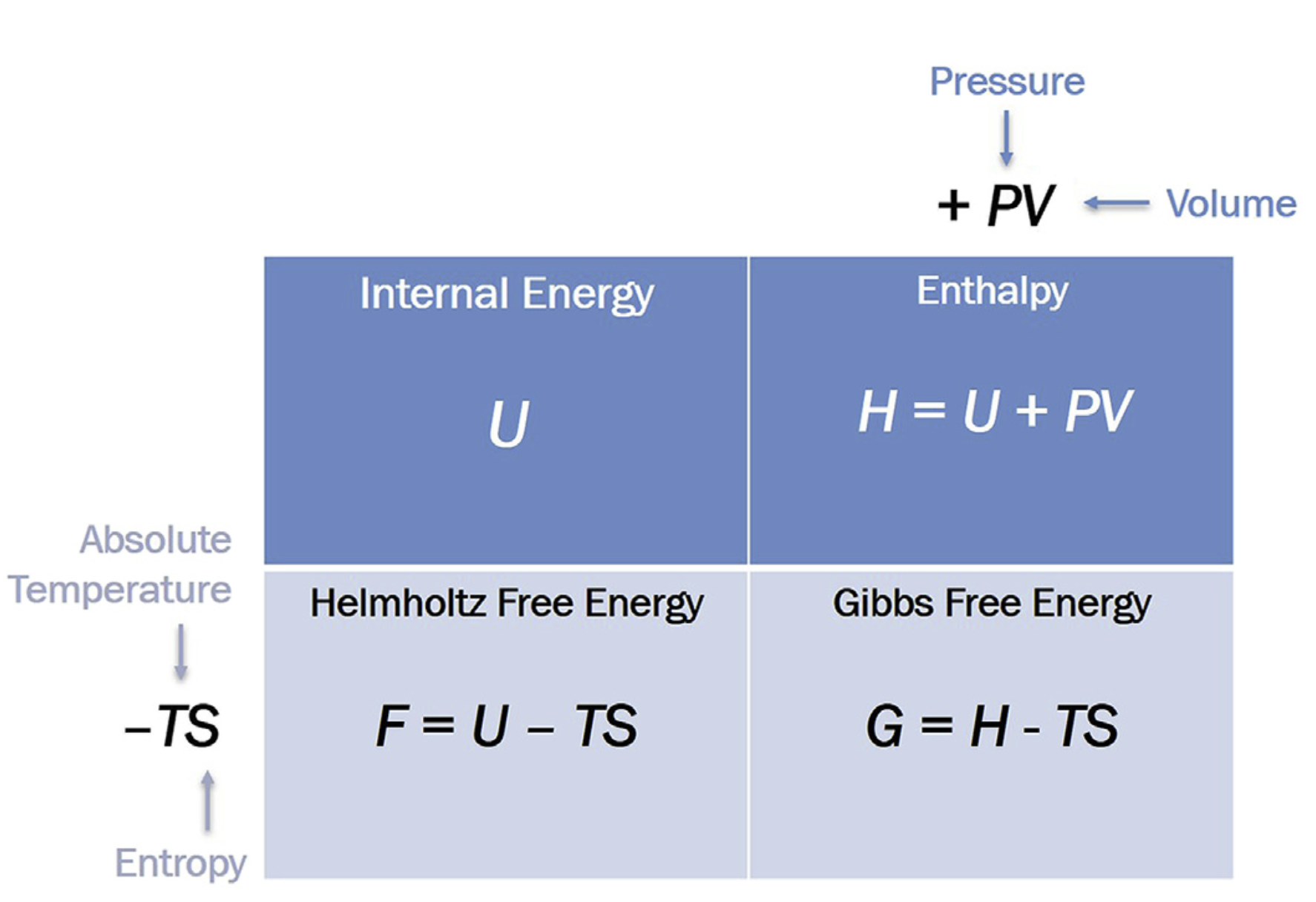
## Thermodynamic Interplay Between \(H\) and \(S\)
Whether a process from A → B is spontaneous or non-spontaneous depends on the sign of free energy difference, \(\Delta G = G_{B} - G_{A}\).
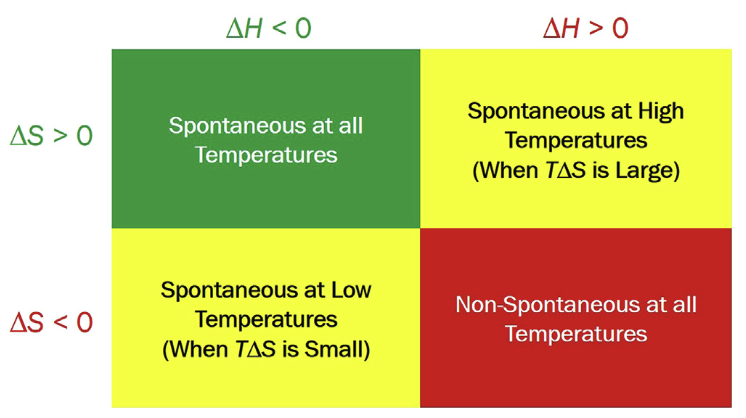
- What is \(\Delta G\) for a reversible process at equilibrium?: \(\Delta G = 0\),
- How can we check the stability of a certain material at \(P, T\)?: Use the phase diagram!
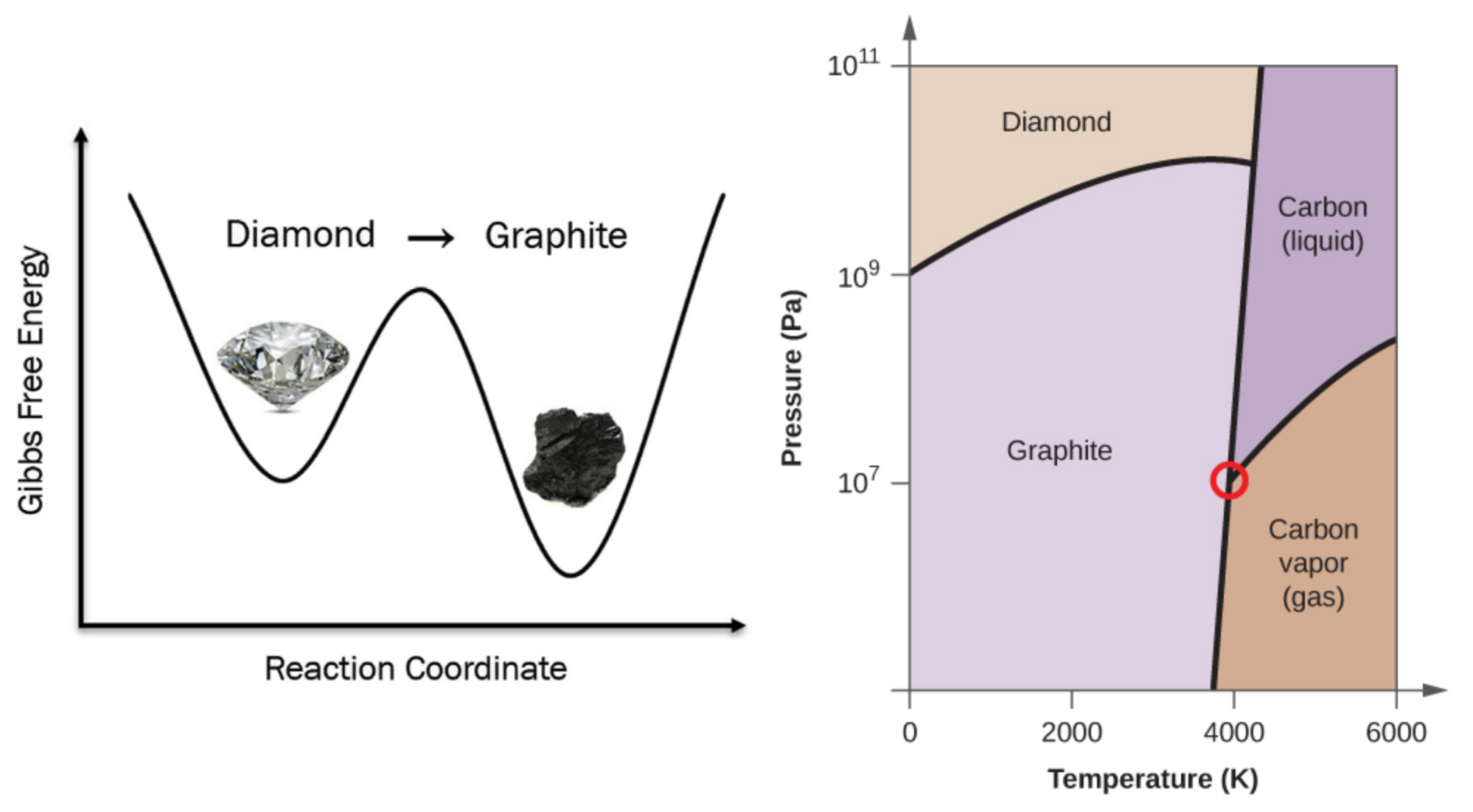
How Stable Is Diamond?
- Which phase is the most stable at r.t. & 1 atm?
- Should we worry our diamond rings turn into pencil?
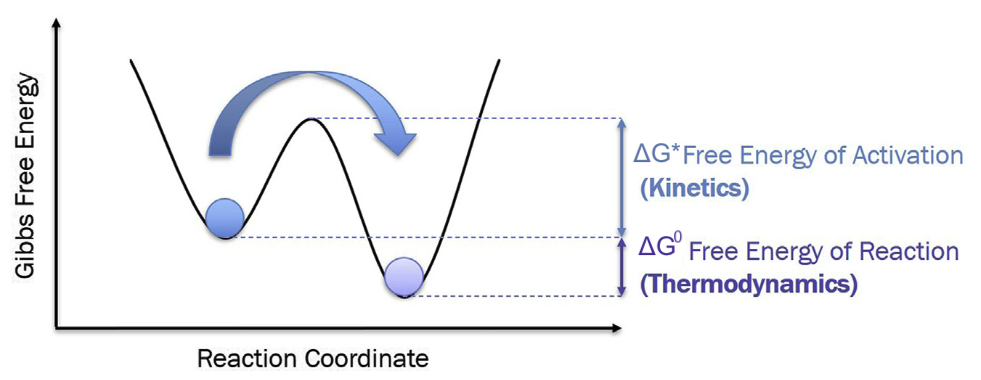
Two-State model of Thermodynamics vs Kinetics
- \(\Delta G^0\): Free energy of reaction → will reaction happen? (Thermodynamics)
- \(\Delta G^*\): Free energy of activation → how likely / fast? (Kinetics)
Arrhenius Plot Demo
Where Does Entropy \(S\) Come From?
- Claussius (1865) Classical thermodynamics.
- Entropy is a state variable of internal energy.
- \(dU(S, V) = TdS - pdV\)
- Boltzmann (1877) Statistical mechanics.
- Entropy is a measure of accessible microstates (atoms + probability!)
- \(S = k_B \log(\Omega)\)
Why The Logarithm?
- \(S\) as an extensive quantity → Additive \(S_T = S_1 + S_2\)
- \(\Omega\) as microstates is multiplicative → \(\Omega_T = \Omega_1 \cdot \Omega_2\)
- If \(S = f(\Omega)\), then \(S_T = f(\Omega_T) = f(\Omega_1 \cdot \Omega_2)\) → \(f(\Omega_1 \cdot \Omega_2) = f(\Omega_1) + f(\Omega_2)\)
- \(f(x) = C \log(x)\) is the unique solution using Cauchy’s functional equation results
Entropy IS NOT Disorder!
- Common statement of entropy is measure of disorder
- Boltzmann equation measures how many possibilities of arrangement
- Disorder is not uniquely linked to number of microstates!
Entropy IS NOT Disorder – II!
- High entropy alloys (HEAs): Example of high configurational entropy material
- Many HEAs have much more ordered lattice than binary alloys
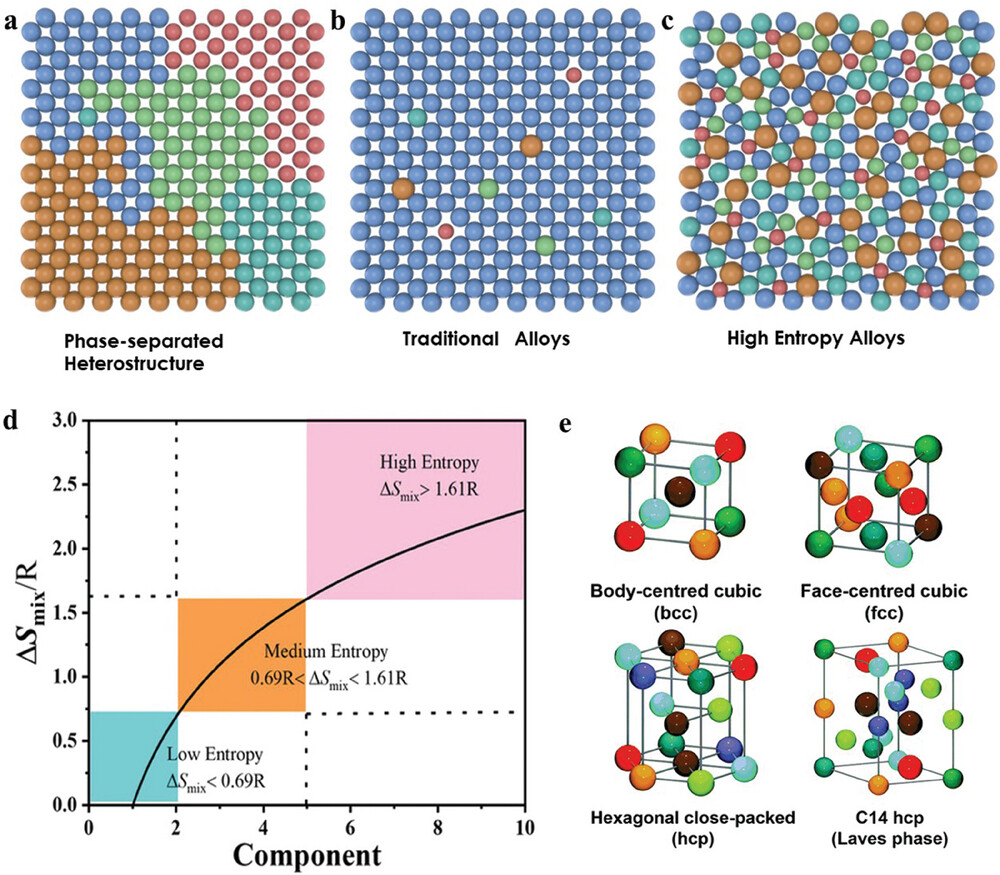
What Should We Really Think of Entropy
- Arrow of time: mixed cream and coffee cannot be demixed
- Newtonian dynamics is time-reversible
- We cannot rewind to low entropy state from Newtonian dynamics!
- Loss of information:
- Shannon entropy: \(S_{info} = -k_B \sum_i p_i \log(p_i)\)
- Shannon entropy can be measured on the exact state!
- Uncertainty:
- Link to Heisenberg’s principle \(\Delta x \Delta p \leq \hbar/2\)
- See Hirschman Am. J. Math., 1957 79, 152
Where Can We Go From Here?
Irreversible thermodynamics
Real processes occur away from equilibrium, where entropy is produced.
Entropy generation as a driving force
Gradients in temperature, concentration, and chemical potential drive fluxes by increasing total entropy.
From equilibrium to dynamics
Entropy provides the unifying language for diffusion, heat flow, chemical reactions, and transport phenomena.
Stay Tuned!
Brief Introduction to Course AI Helper
- A Socratic Gemini chatbot aiming to help course learning and key concepts
- Access the AI helper here: 👉 https://gemini.google.com/gem/1c118102b2d1
Summary
What we learned today:
- Syllabus / course contents of MATE 664
- Kinetic rate and equilibrium
- Concept of entropy revisited
- Laws of thermodynamics revisited
See you next time!
